Background: We have previously reported the success of treating children with Burkitt lymphoma (BL) resulting in a ≥90% long-term EFS utilizing short intensive rituximab containing chemoimmunotherapy (Cairo et al, Blood, 2007; Goldman /Cairo et al, Leukemia, 2012, Goldman /Cairo et al, Br J Haematol., 2014; Goldman /Cairo et al, Leukemia, 2021). However, the prognosis is dismal in children who are refractory or progress after chemoimmunotherapy (Cairo et al Br J Haematol. 2018). The mechanisms associated with this poor prognosis are mainly due to chemoradioimmunotherapy resistance and an immunosuppressive BL microenvironment (TME) (Cairo et al Br J Haematol. 2016; Cairo et al Br J Haematol. 2019) and represent an urgent unmet need. The CD20 molecule is universally expressed by normal B cells in all stages of development, from the pre-B cell up to the mature plasma cell as well as by most B cell malignancies including CLL, FL and BL (Chu/Cairo, BJH, 2016). Obinutuzumab is a humanized, type II anti-CD20 monoclonal antibody glycoengineered to enhance Fc receptor affinity. It has lower complement-dependent cytotoxicity than rituximab but greater ADCC, phagocytosis and direct B-cell killing effects (Chu/Cairo, BJH, 2018). Obinutuzumab has been successfully utilized in front-line therapy in FLL (Marcus, et al, NEJM, 2017) and CLL (Goede, et al, NEJM, 2014; Moreno, et al, Lancet, 2019). Our group has successfully ex-vivo expanded functional and active peripheral blood NK cells (PBNK) with irradiated feeder cells APC (K562-IL21-41BBL) (Chu/Cairo, et al, JITC 2020). We previously demonstrated that obinutuzumab has significantly enhanced ex-vivo expanded PBNK mediated cytotoxicity against BL and pre-B-ALL cell lines compared to rituximab (Tiwari/Cairo et al, BJH, 2015). NKTR-255 is an IL-15 receptor agonist designed to activate the IL-15 pathway and NK cells and promote the survival and expansion of memory CD8+ T cells without inducing suppressive regulatory T cells (Kuo/Zalevsky, Cancer Res. 2017). NKTR-255 stimulates proliferation and survival of NK, CD8+ T cells, which may lead to sustained anti-tumor immune response.
Objective: To investigate the anti-BL effects of NKTR-255 on the ADCC of ex-vivo expanded NK cells with obinutuzumab against rituximab-resistant BL in vitro and in vivo using human BL xenografted NSG mice.
Methods: NK cells were expanded with lethally irradiated K562-mbIL21-41BBL cells as previously described (Denman/Lee, et al, PLoS One, 2012). Expanded PBNK cells were isolated using Miltenyi NK cell isolation kit. NKTR-255 was generously provided by Nektar Therapeutics. In vitro cytotoxicity was examined using luminescence reporter-based assays. IFN-g, granzyme B and perforin levels were examined by standard enzyme-linked immunosorbent assays as we previously described (Chu/Cairo, Front. Immunol, 2022). Rituximab-resistant BL cells Raji-2R and Raji-4RH were used as target cells. Luciferase expression Raji-4RH cells were xenografted to NSG mice as we previously described (Chu/Cairo, et al, JITC 2021).
Results:
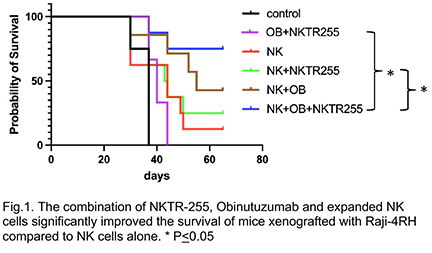
NKTR-255 significantly enhanced the proliferation of ex-vivo expanded NK cells (p<0.0001) at day 7. NKTR-255 significantly enhanced the in vitro cytoxicity of expanded NK cells when combined with obinutuzumab against rituximab-resistant BL cells like Raji-2R (E:T=3:1, p <0.01), and Raji-4RH (E:T=3:1, p<0.01) as compared to the control groups. NKTR-255 also significantly enhanced IFN-g, granzyme and perforin release from expanded NK cells when combined with obinutuzumab against Raji-2R (E:T=3:1, IFN-g: p<0.001, granzyme: p<0.001 and perforin: p<0.001) and Raji-4RH (E:T=3:1, IFN- g: p<0.001, granzyme: p<0.01 and perforin: p<0.01) as compared to controls. Our in vivo study demonstrated that the combination of NKTR-255, Obinutuzumab and expanded NK cells significantly improved the survival of mice xenografted with Raji-4RH compared to controls (Fig.1).
Conclusion:
We found that NKTR-255 significantly enhanced the ADCC of expanded NK cells with Obinutuzumab against rituximab-resistant BL cells in vitro with enhanced IFN- g, granzyme B and perforin release. The in vivo effects of NKTR-255 with expanded NK cells and Obinutuzumab against rituximab-resistant BL cells using humanized NSG models are very promising. Mechanisms studies of BL relapsed from the combination therapy are under investigation. (Supported by HHOW and St Baldrick grants).
Disclosures
Lee:Kiadis Pharma, a Sanofi Corporation: Consultancy, Patents & Royalties: licensed through Nationwide Children's Hospital; Avidicure B.V.: Consultancy, Current equity holder in private company, Research Funding. Marcondes:Nektar Therapeutics: Current Employment. Klein:Roche/Genentech: Current Employment, Current equity holder in publicly-traded company, Other: Stock ownership, Patents & Royalties. Cairo:Sanofi: Honoraria, Speakers Bureau; Sobi: Honoraria, Speakers Bureau; Merck: Research Funding; Miltenyi Biotec: Research Funding; Novartis: Consultancy; Servier Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen Inc.: Honoraria, Speakers Bureau; Abbvie: Consultancy; Celularity: Research Funding; Astra Zeneca: Honoraria; Omeros Pharmaceuticals: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal